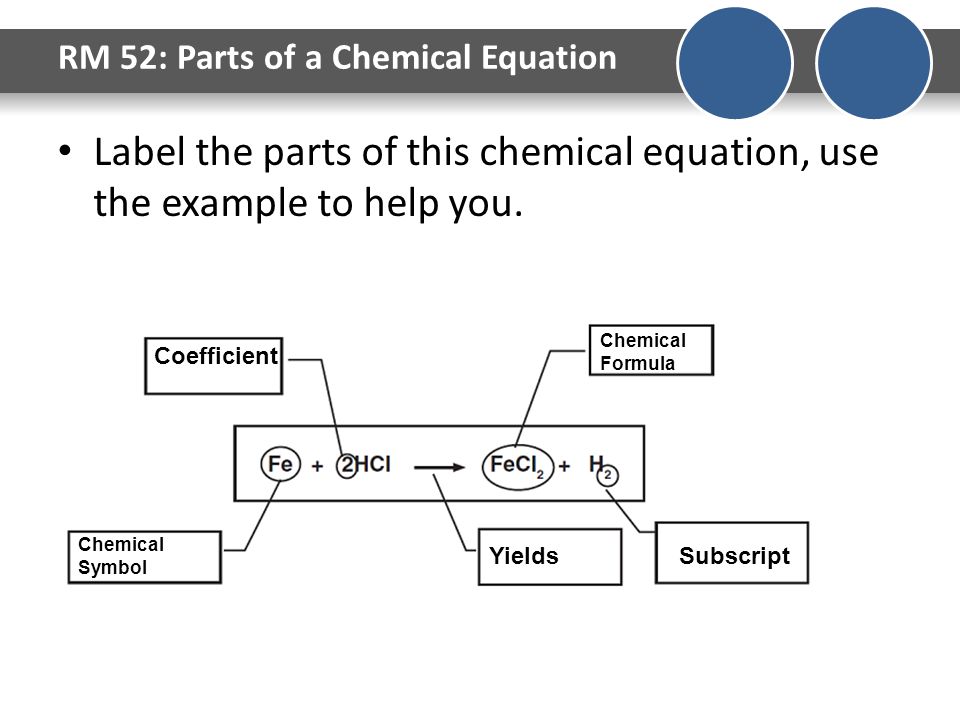
43 label a chemical equation
Chemical Equations Flashcards | Quizlet Chemical Reaction. process in which bonds between atoms are broken and new bonds are formed. Coefficient. indicates the # of each type of molecule (# that multiplies a term in an equation). ex. 6H2O means there are six water molecules. Subscript. the small 2 (subscript) in H2O is the # of hydrogen atoms. Reactant. What are Chemical Equations? Detailed Explanation, Examples Chemical Equation: CaCl 2 + 2AgNO 3 → Ca(NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3 - → Ca 2+ + 2NO 3 - + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ (calcium ion) and the NO 3 - (nitrate) ions are present on both sides of the ionic equation. These ions are referred to as spectator ions because they do not participate in the chemical reaction.
Chemical Equations - Let's Talk Science For a balanced chemical equation, the number of atoms on the left side of a chemical equation has to equal the number of atoms on the right side of the equation. We started off with H 2 (g) + O2 (g) on the right-hand side of the equation. This means that there are two atoms of hydrogen (H 2) and two atoms of oxygen (O 2 ): H2 (g) + O2 (g)
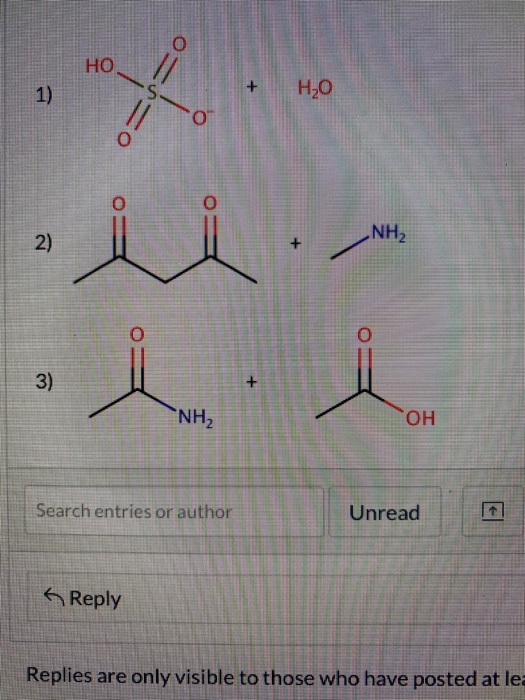
Label a chemical equation
How to write a chemical formula? - TeX - Stack Exchange In order to produce the following output involving a chemical formula. I can attempt to write the chemical formula as a mathematical formula: \documentclass {report} \usepackage {amsmath} \begin {document} As the scintillator $\text {PbWO}_ {\text {4}}$ crystals are used. \end {document} However, this method is a brute force when approaching a ... 3 Steps for Balancing Chemical Equations - ThoughtCo 1) Write the unbalanced equation. SnO 2 + H 2 → Sn + H 2 O Refer to Table of Common Polyatomic Ions and Formulas of Ionic Compounds if you have trouble writing the chemical formulas of the products and reactants. 2) Balance the equation. Look at the equation and see which elements are not balanced. Chemical Equation | Reactants And Products In Chemical Reactions The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation. Law of conservation of mass governs the balancing of a chemical equation. According to this law, mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the elements or molecules present on the reactant side should be equal to the total mass of elements or molecules present on the product side. If the number ...
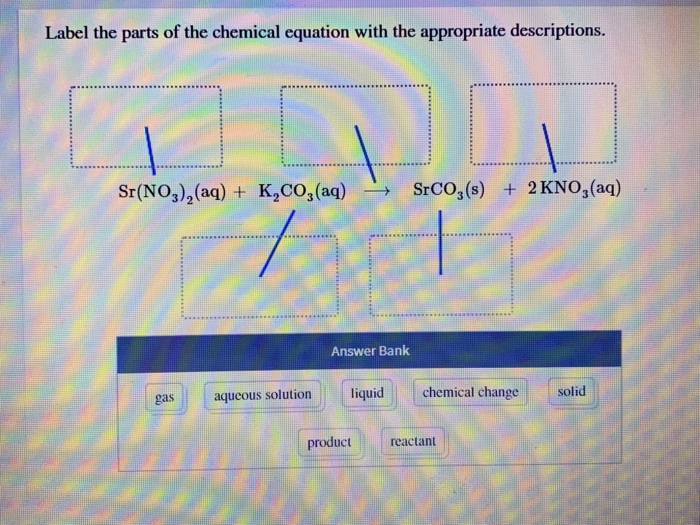
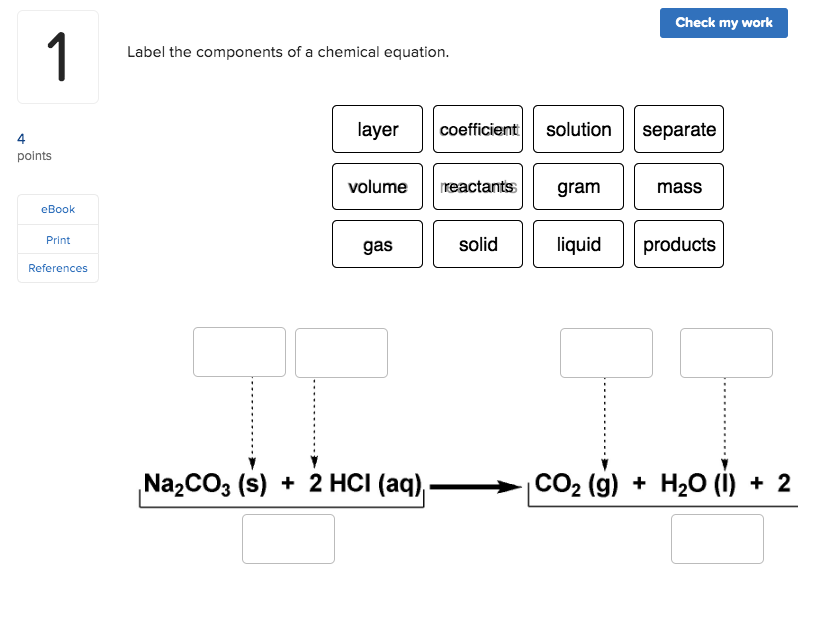
Label a chemical equation. Solved Label the components of a chemical equation. separate | Chegg.com Expert Answer. 100% (49 ratings) Transcribed image text: Label the components of a chemical equation. separate mass liquid coefficient gas solution solid reactants layer prod uiets volume gram Na2co, (s) + 2HCl (aq),- ?.co2 (g) + H2O (l) Co2 (g) + H20 ) + 2 Naci (aq) Previous question Next question. How to Write a Chemical Equation (with Pictures) - wikiHow In a basic double replacement equation you will have 2 cations and 2 anions. The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. [11] For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2 Symbols used in Chemical Equations Flashcards | Quizlet chemical equation an expression representing a chemical reaction skeleton chemical equation does not indicate the relative amounts of reactants and products. (frame) Law of Conservation of Mass in any physical change or chemical reaction, mass is conserved. Mass can be neither created nor destroyed, simply rearranged. catalyst Symbols in Chemical Equations - Harper College Symbols in Chemical Equations. Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read used when the reaction can proceed in both directions - this is called an equilibrium arrow and will be used later in the ...
Solved Draw and label a balanced chemical equation for the | Chegg.com Label the conjugate acid and conjugate base in your products. Determine the direction of the reaction (whether; Question: Draw and label a balanced chemical equation for the acid-base reaction between the following pairs of molecules. Your balanced chemical equation should include: Label the acid and base in the reaction. Labeling A Chemical Equation Part 2 - YouTube Labeling A Chemical Equation Part 2 - YouTube. Examples of Balanced Chemical Equations - ThoughtCo When you balance a chemical equation, it's always a good idea to check the final equation to make sure it works out. Perform the following check: Add up the numbers of each type of atom. The total number of atoms in a balanced equation will be the same on both sides of the equation. How would you label each formula in the chemical equation below as ... Explanation: The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron, F e +S → F eS. Iron and sulfur are the reactants, and iron sulfide is the product.
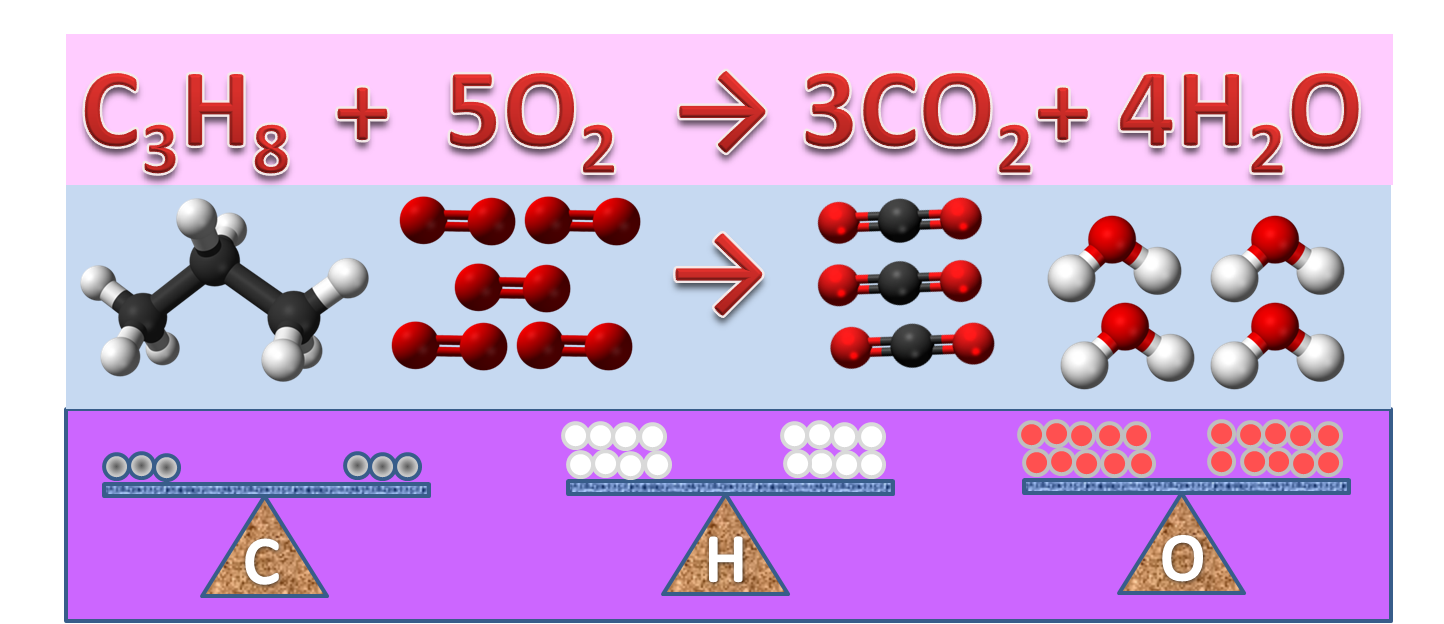
Parts of a chemical Equation- Write an equation and label the following ... Coefficient, subscript, Atoms, molecules, Reactants, and products are parts of a balanced chemical equation Balanced chemical equation 6CO₂ ₊ 6H₂O=1C₆H₁₂O₆₊61O₂ Coefficient: A multiplier or factor that measures some property. Here 6,6,1 ad 6 are the Coefficient of CO, H₂O, C₆H₁₂O₆, and O₂ respectively. Chemical equation - Wikipedia a chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and … How do you label a chemical equations? - Answers Wiki User. ∙ 2013-01-28 13:17:07. Study now. See answer (1) Best Answer. Copy. N2 + h2 =nh3. Wiki User. ∙ 2013-01-28 13:17:07. PDF Laboratory Safety Labeling and Transfer Facts of Chemicals of Chemicals Permanent Container Labels . Employers must ensure that no worker uses, stores, or . allows any other person to use or store any hazardous . substance in a laboratory if the container (including . bags, barrels, bottles, boxes, cans, cylinders, drums . and reaction vessels) does not meet the following . labeling requirements in ...
How do you Write a Chemical Equation? - A Plus Topper Chemical equations give us the following qualitative information. (a) Reactants and products of a chemical reaction. (b) Physical states of the reactants and products. Take the following equation as an example. 2C(s) + O 2 (g) 2CO(g) From the equation, we know that the reactants are solid carbon and oxygen gas. The product of the reaction is ...
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Start by writing the chemical equation in terms of the substances involved: C 2 H 6 + O 2 → CO 2 + H 2 O We have two carbon atoms on the left, so we need two carbon dioxide molecules on the product side, so that each side has two carbon atoms; that element is balanced.
What is a Chemical Equation? - Definition & Examples Chemical equations tell us the elements and/or compounds that are reacting and what the product (s) of the reaction. The coefficients on the substances in the reaction tell us the mole ratio or...
How to Write Chemical Equations - YouTube Mr. Causey shows you how to WRITE chemical equations. Mr. Causey discusses the parts of a chemical equation, the symbols involved and the steps required to w...
Chemical Ingredients 101: How to Read a Product Label How to read a chemical label. The U.S. Occupational Safety and Health Administration (OSHA) requires hazardous chemical labeling as a part of its recent revision of the Hazard Communication Standard. All labels are required to have the following: Pictograms; A signal word; Hazard and precautionary statement; The product identifier; Supplier identification
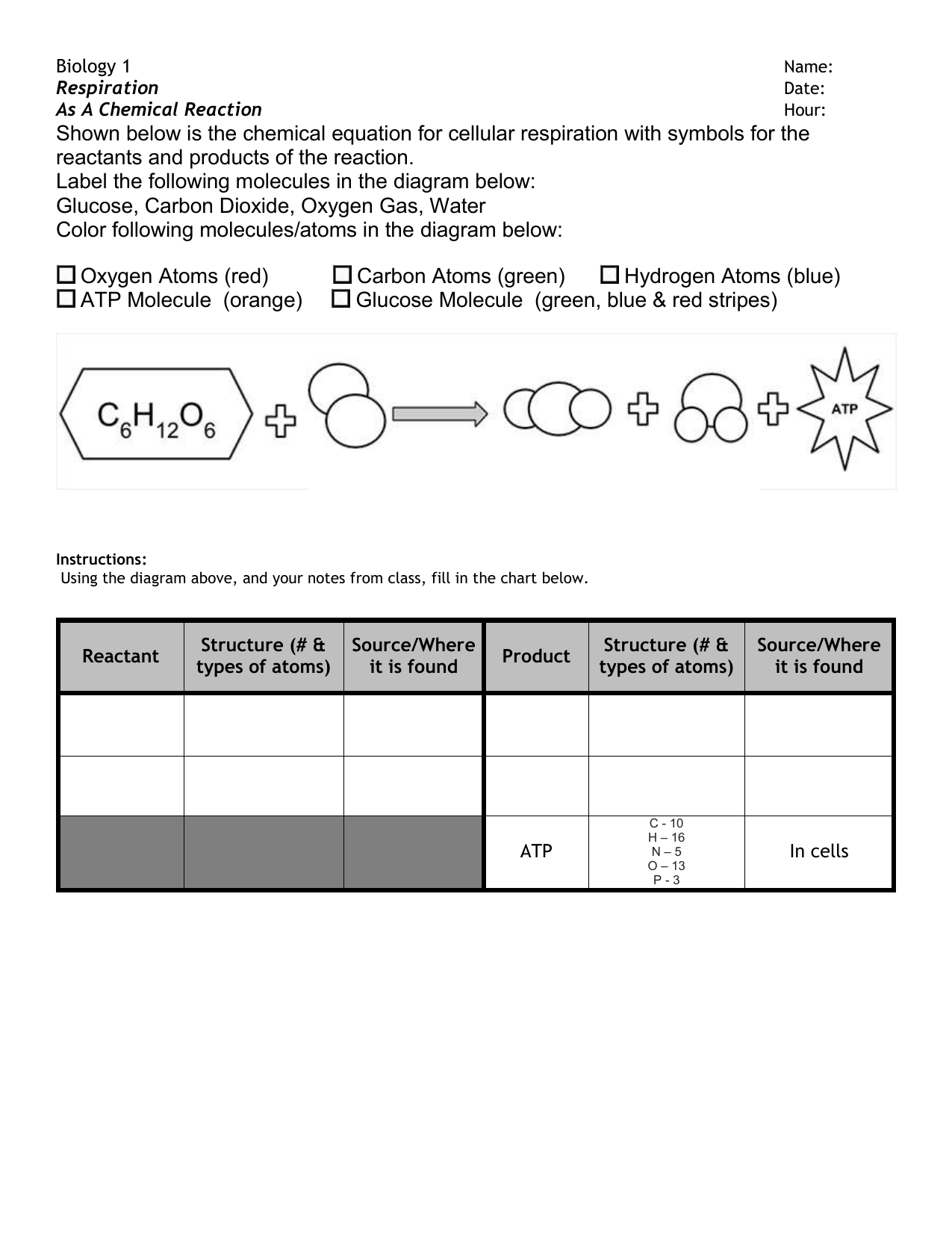
Using Moles in Chemical Equations | Study.com Here is the chemical equation for making water: We see that 2 molecules of hydrogen reacts with 1 molecule of oxygen to form 2 molecules of water. Now typically we aren't only making 1 molecule...
label the parts of a chemical equation - ds-bud.pl A chemical equation consists of the chemical formulas of the reactants (the starting substances) and the chemical formula of the products (substances formed in the chemical reaction). The two are separated by an arrow symbol ( → {displaystyle rightarrow }, usually read as "yields") and each individual substance's chemical formula is separated ...
What are the Parts of a Chemical Equation? - Life Persona 2 - Begin by writing the chemical equation in terms of the substances involved: C 2 H 6 T the 2 → CO 2 + H 2 OR We have two carbon atoms left, so we need two molecules of carbon dioxide on the side of the product, so that each side has two carbon atoms. That element is balanced.
Chemical Equation | Reactants And Products In Chemical Reactions The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation. Law of conservation of mass governs the balancing of a chemical equation. According to this law, mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the elements or molecules present on the reactant side should be equal to the total mass of elements or molecules present on the product side. If the number ...
3 Steps for Balancing Chemical Equations - ThoughtCo 1) Write the unbalanced equation. SnO 2 + H 2 → Sn + H 2 O Refer to Table of Common Polyatomic Ions and Formulas of Ionic Compounds if you have trouble writing the chemical formulas of the products and reactants. 2) Balance the equation. Look at the equation and see which elements are not balanced.
How to write a chemical formula? - TeX - Stack Exchange In order to produce the following output involving a chemical formula. I can attempt to write the chemical formula as a mathematical formula: \documentclass {report} \usepackage {amsmath} \begin {document} As the scintillator $\text {PbWO}_ {\text {4}}$ crystals are used. \end {document} However, this method is a brute force when approaching a ...









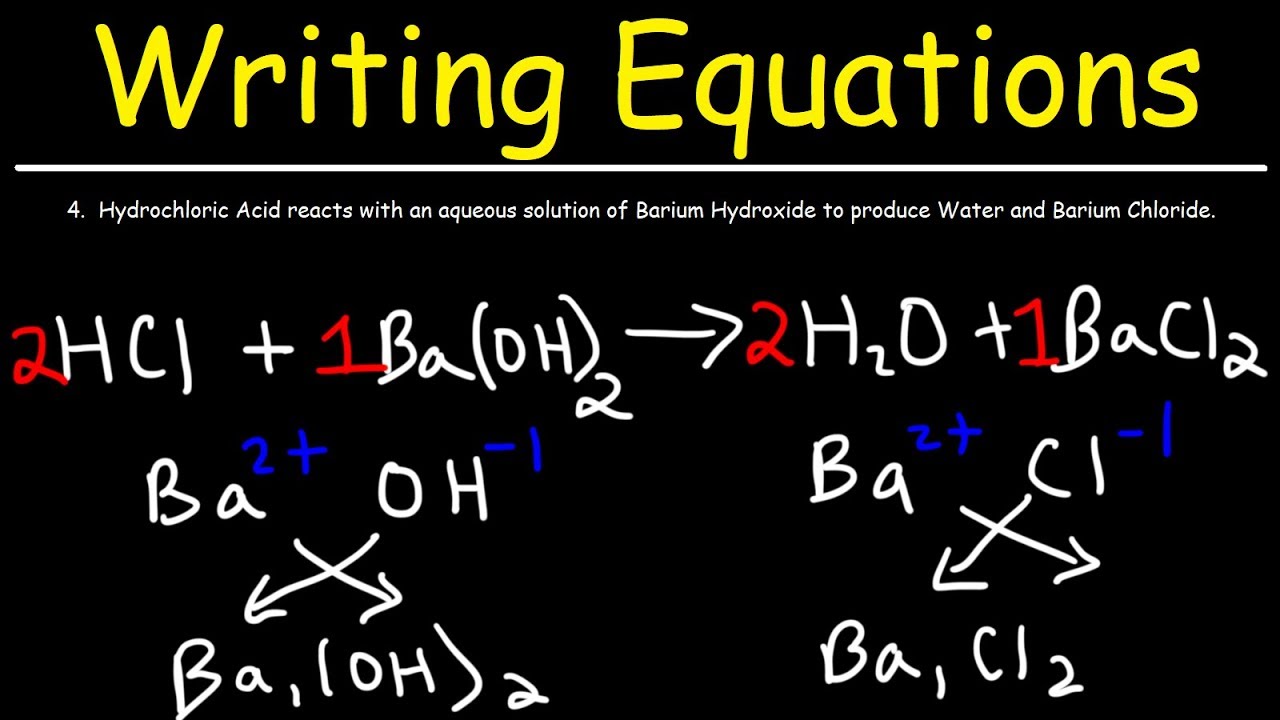
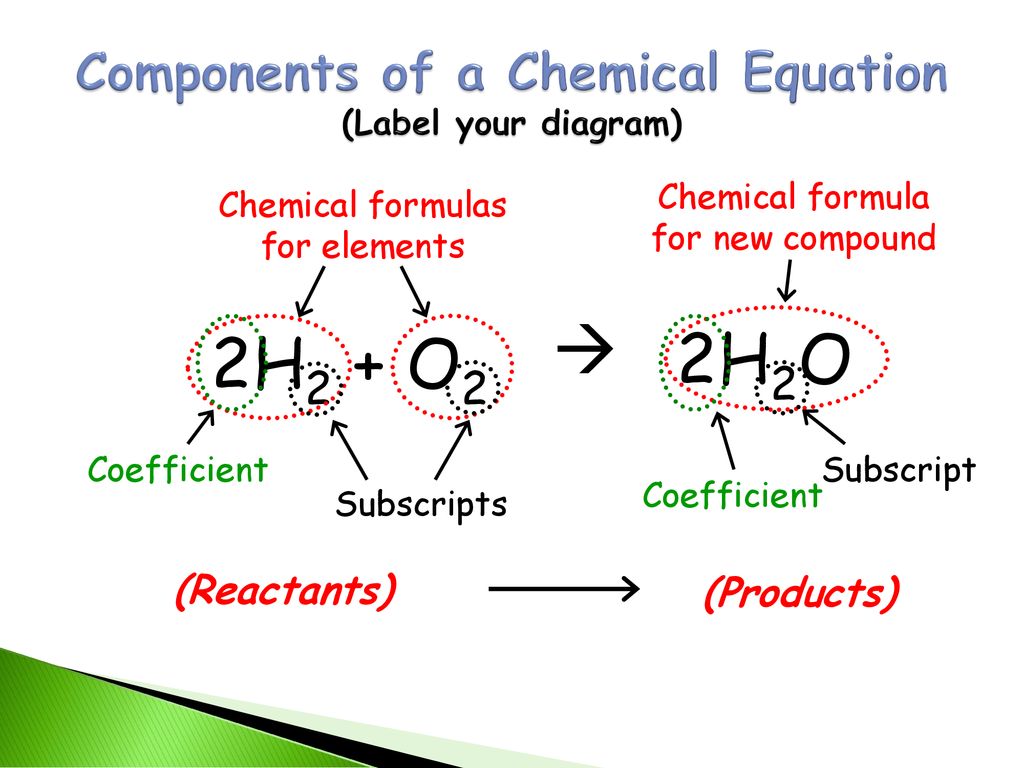


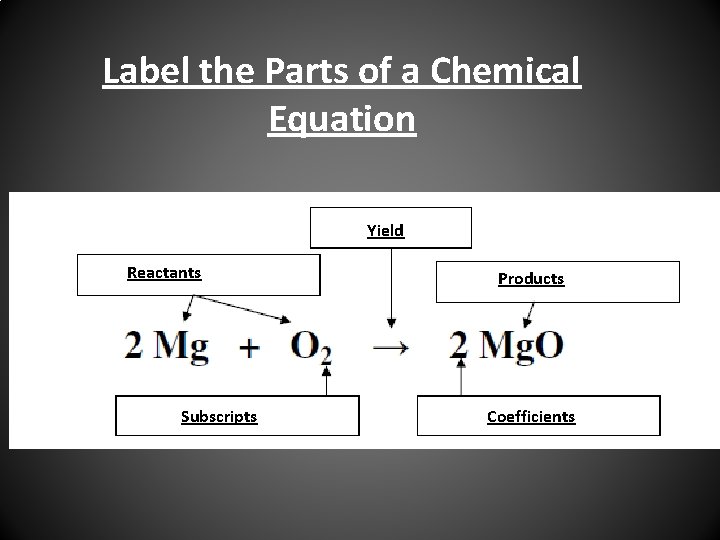











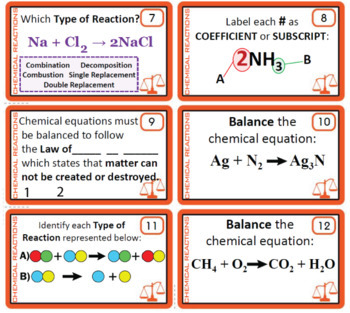



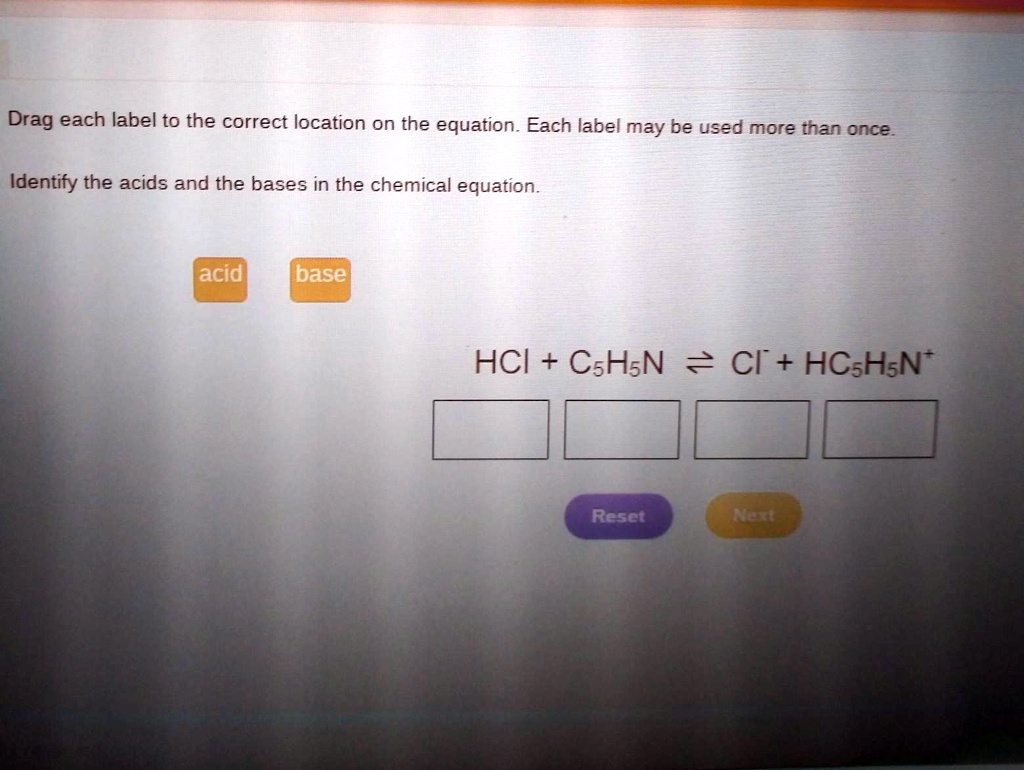

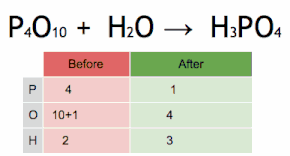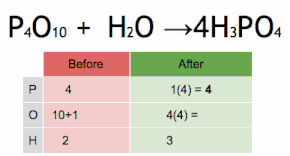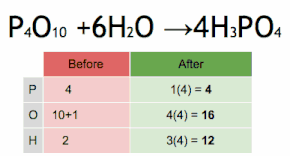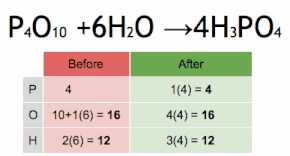
Post a Comment for "43 label a chemical equation"